Ensuring GxP Compliance with Tableau Cloud: Best Practices and Strategies
A comprehensive roadmap to regulatory adherence.

Disclaimer: This white paper is intended solely for informational purposes and should not be interpreted as legal, or professional advice. The content is based on insights gained from interactions with leading global pharmaceutical companies running validated Tableau Cloud environments. While we strive to provide accurate and reliable information, Wiiisdom nor Salesforce cannot be held liable for any inaccuracies or omissions. It is a set of good practices for GxP good practices!
Executive Summary
Healthcare and Life Sciences (HLS) companies operate in one of the most highly regulated industries in the world. Ensuring the proper research, development, and manufacturing of drugs and medical devices is crucial for providing patients with safe, swift, and effective treatment. GxP or Good x Practices, is a series of quality guidelines and practices applied to computerized systems like Tableau. It is enforced by regulatory agencies like the FDA to ensure that life science products are properly made. Ultimately, everyone should appreciate that HLS companies are subject to stringent regulations to mitigate risks, as these guidelines ensure the delivery of safe and effective products for the health of all.
For years, HLS companies have depended on Tableau Server to make informed decisions, ensure accurate results, and administer life-saving treatments, while complying with GxP guidelines (implementing key practices and controls). However, the advent of artificial intelligence (AI) has significantly transformed the industry. This paradigm shift, coupled with overall modernization drives, has urged many HLS companies to prepare for a transition to the Cloud, enabling them to leverage cutting-edge innovations such as Generative AI Analytics (GenAI Analytics) as part of their corporate strategy to reduce costs and enhance the efficiency of drug and device delivery in a highly competitive environment.
Tableau Cloud is at the forefront of this transformation, investing in AI-driven solutions for the future, in a fully-hosted, cloud-based, enterprise-grade analytics solution. With tools like Tableau Agent and Tableau Pulse, and innovations like the Einstein Trust Layer, Tableau Cloud is democratizing data analysis and streamlining the consumption of insights at scale. To leverage these advanced capabilities, HLS companies must first transition from Tableau Server to Tableau Cloud. However, moving to and running Cloud computing brings challenges in the context of GxP compliance to these companies. The existing key practices and controls in place to ensure GxP compliance for Tableau Server will not work in a Cloud environment. For example, on average, it takes HLS customers six months to test (including platform qualification and business validation) Tableau Server upgrades while Tableau Cloud is upgraded many times a year. This white paper aims to list all the challenges, as well as provide new key practices and controls to overcome them.
Challenges of GxP Compliance Moving to Tableau Cloud
The Migration to Tableau Cloud
HLS companies looking to leverage GenAI capabilities must migrate from Tableau Server to Tableau Cloud, a process that in itself presents several challenges, especially for validated environments.
Qualifying and Validating Tableau
Preparing the Tableau Cloud platform for GxP compliance is critical in ensuring a smooth migration process. It’s important to be familiar with Tableau Cloud’s platform and system qualification1 documentation in the Salesforce Trust Center, along with their guidance specific to companies subject to GxP. This also includes defining the key roles and responsibilities for this project.
Challenges of GxP Compliance Running Tableau Cloud
Tableau Cloud Upgrades
Once in Tableau Cloud, and as in any cloud-based solution, customers lose control over upgrades, and this poses a significant obstacle, especially for highly-regulated deployments. HLS companies often name cloud-based solution upgrades as “forced” upgrades. Currently, with Tableau Server, companies have complete control over whether to apply upgrades or not, which is seen as a major barrier to migrating to Tableau Cloud in the context of GxP.
Key Practices and Controls to Ensure Tableau Cloud GxP Compliance
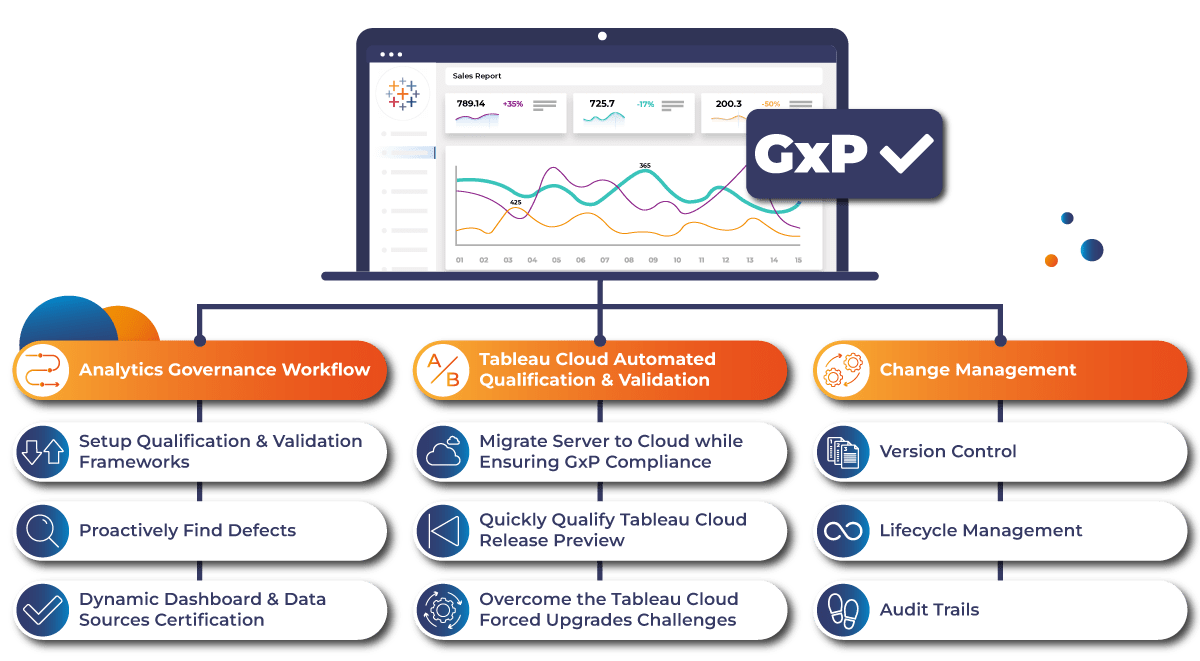
There is a real need to modernize key practices and controls currently used to ensure GxP compliance on Tableau Server. One of the key components is automation.
HLS companies should focus on implementing three key qualification and validation frameworks while running Tableau Cloud:

Automated Testing with Related Audit Trails

Continuous Monitoring with Related Audit Trails

Change Management with Related Audit Trails
Automated regression testing and related documentation must be used to test the migration, test any new version (coming with upgrades), and proactively test major releases using Tableau Cloud Release Preview sites. For HLS companies, timing and speed are critical. The faster they complete qualifications, the more time they have to address potential defects (bugs, regressions, side effects).
Automated testing and related documentation must also be used for validation purposes to ensure the quality of dashboards and underlying Tableau data sources, completeness, accuracy, and reliability.
Periodic reviews of Tableau Cloud artifacts should be conducted to ensure they remain in a validated state, which involves constant validation or revalidation of the systems. Automated testing during migrations, upgrades, or to validate major releases using Release Previews sites is not sufficient. In a Cloud environment, continuous monitoring and testing are essential.
It’s critical to set up a solid Tableau content lifecycle management. HLS companies may already have a similar workflow in place on Tableau Server that needs to be adapted as the Tableau Cloud production sites will be flooded with new content or new versions of existing content. Implementing robust change management2 practices, including enterprise-grade version control and associated audit trails, is essential to track modifications and maintain the integrity of systems.
The Benefits of GxP Compliance
HLS companies are well-positioned to successfully adopt Tableau Cloud and unlock AI analytics due to their unwavering commitment to quality, fostered by GxP guidelines. While some may view GxP as a constraint, for HLS companies, it is an invaluable asset that ensures high-quality analytics assets, thereby minimizing AI hallucinations, particularly in Tableau data sources, which are central to many of Tableau AI’s innovations.
Mastering GxP compliance also enables HLS companies to apply best practices to other regulations, such as SOX, privacy, and healthcare compliance. Additionally, implementing these GxP best practices for Tableau can benefit other regulated industries, including financial institutions and public companies, by enhancing their regulatory compliance and Tableau capabilities.
Why Partner with Wiiisdom
Adopting Wiiisdom solutions allows HLS companies to achieve quick, impactful wins across the entire data journey, from analytics consumption to Tableau AI insights, fostering a culture of quality and informed decision-making. Being a trusted technology Tableau partner, we complement Tableau with innovative Analytics Governance solutions to enable the frameworks mentioned earlier. This is through technology that automates testing and validation, lifecycle management, continuous monitoring, versioning and traceability, and patent pending dynamic and automated content certification. Wiiisdom can help meet and exceed GxP expectations, while also ensuring the accuracy of all business-critical decisions. Wiiisdom gained unique expertise by partnering with the largest pharmaceutical companies in the world to address GxP while running Tableau Cloud (as well as Tableau Server), serving tens of thousands of Tableau users.
Demonstrating GxP Compliance with Tableau Cloud
HLS companies leverage Tableau in digitizing essential processes, including drug and device development, pharmacovigilance reporting, and regulatory compliance activities. For years, HLS companies have relied on Tableau Server to make confident decisions, deliver accurate results, and provide life-saving treatments. To harness the power of the Cloud and GenAI, HLS companies must swiftly transition to Tableau Cloud, following the lead of their industry peers.
Tableau offers its Tableau Cloud platform and innovations as a Software as a Service (SaaS) solution, which introduces a shared responsibility model. Demonstrating GxP adherence while running Tableau Cloud becomes different but not necessarily harder compared to the on-premise Tableau Server. Software, whether on-premise or SaaS, should be developed and managed through formal processes, ensuring it is fit-for-purpose and aligns with industry best practices and standards to ensure quality, compliance, and IT security.
Under the Cloud-shared responsibility model, HLS companies inherit certain administrative, technical, and physical security controls from Tableau. These controls, particularly those relevant to Tableau Cloud qualification, are extensively documented in Tableau’s various security framework attestations. Despite this inheritance, HLS companies must demonstrate that Tableau Cloud is used in a validated state, meaning they must manage, demonstrate, and prove control over Tableau Cloud.
By achieving GxP compliance with Tableau Cloud, HLS companies can reap numerous benefits while leveraging Tableau’s latest innovations in GenAI capabilities and aligning with corporate strategies. Tableau empowers users with data, delivering faster analytics at scale. Leading HLS organizations can achieve greater agility, accelerated time to value, and operational efficiencies by combining the power of the Cloud with the world’s leading analytics platform.
All the principles listed in this white paper are applicable to Tableau Cloud but remain relevant if you are running Tableau Server.
Challenges of GxP Compliance
The Imperative of a Full Migration to Tableau Cloud
Pharmaceuticals, Biotech, Medical Devices, Healthcare, and Life Sciences (HLS) companies have relied on Tableau Server with the necessary qualification and validation processes. However, maintaining a hybrid environment, where only non-validated portions move to the Cloud while validated portions remain on-premise, is costly and leads to a fragmented user experience. Users might benefit from innovations like Tableau Pulse for sales insights but miss out on similar advancements for pharmacovigilance. AI and innovation are crucial for validated environments, making it essential for HLS companies to fully migrate to Tableau Cloud. This ensures seamless access to advanced analytics and generative AI capabilities, enhancing operational efficiency and compliance. HLS and particularly, Life Science, is acknowledged as the industry that stands to gain the most from GenAI.
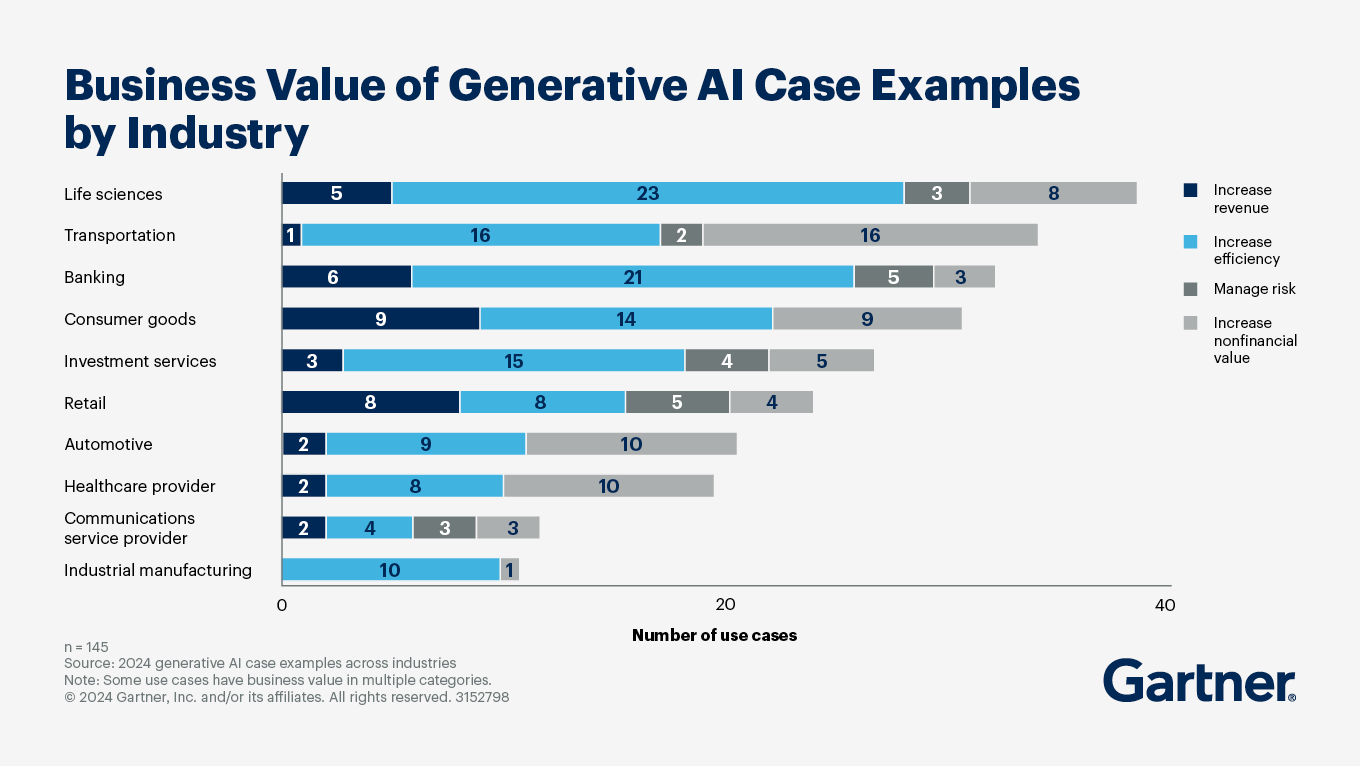
Source: https://emt.gartnerweb.com/ngw/globalassets/en/articles/images/business-value-of-generative-ai-case-examples-by-industry.png?_gl=1*1ttlxak*_ga*NzIzNjE4NjI1LjE3MDkyMjMyOTk.*_ga_R1W5CE5FEV*MTczODAxNzE5OC4zMS4wLjE3MzgwMTcyMDEuNTcuMC4w*_gcl_au*MTQ5NjEzNTM0NC4xNzM1ODIzODM2
The validated footprint is particularly significant. Our interactions with HLS-pharmaceutical customers and prospects reveal that, on average, 15 to 25% of the Tableau Server platform is fully validated. The remaining 75 to 85% is primarily utilized for operational reporting, business development, and other non-validated activities.
You might already have a hybrid Tableau deployment, with the non-validated portion on Tableau Cloud and the validated portion on Tableau Server. The recommendations in this white paper still remain applicable, though you may need to slightly adjust your migration plan.
NB: If your current Tableau Server deployment includes data and users in China, you will need to keep this part on-premise as it cannot be hosted on Tableau Cloud/AWS.
The Challenges of Tableau Cloud Forced Upgrades
It is evident that when you move to Tableau Cloud, there is a technical reason to qualify and validate everything. The main challenge with Tableau Cloud is not the migration itself, which is a one-time project, but rather the further functional changes that may occur during upgrades, also known as releases, to Tableau Cloud. Today while running Tableau Server, you have control over the application or not of an upgrade, i.e. you can literally hold a Tableau Server upgrade until everything is fully validated. Such control is not available in Tableau Cloud and this is seen as a major blocker for any GxP-compliant Tableau Cloud migration.
One of the largest pharmaceutical companies (>100k users) is currently moving to Tableau Cloud, but the upgrades pose a critical challenge. Their testing timelines may not allow enough time for full validation before each monthly upgrade. Additionally, their current process is to remediate issues before going live and will hold any new deployment or version until everything is validated. However, this becomes a critical challenge in Tableau Cloud because Tableau takes care of the updates, monitoring, and infrastructure maintenance.
Read 👉 Overcome the challenges of Tableau Cloud forced upgrades with Wiiisdom
On average, it takes HLS customers six months to test (including platform qualification and business validation) when you upgrade Tableau Server. There is a real need to modernize Analytics Governance practices, including testing, lifecycle management, and continuous monitoring at scale with automation. Following the qualification and validation frameworks in this white paper can help.
Despite the challenges, upgrades in Tableau Cloud introduce significant enhancements, making the platform faster, more secure, more efficient, and more effective. This may require revalidation but offers additional business benefits. Adhering to your change management process, including version management and maintaining an audit trail, is essential when updating dashboards or data sources when new functionality is introduced.
Tableau Cloud Platform Readiness
Tableau Cloud Platform/System Qualification
Tableau Cloud meets global SaaS data security standards such as ISO 27001/27017/27018 and SOC 1/2 and complies with GDPR. For GxP compliance, regulated companies must take responsibility, leveraging Tableau Cloud’s quality controls and secure development practices. Key features include the Activity Log for auditing and stringent data security protocols. Comprehensive documentation and compliance certifications can be accessed through the Salesforce Trust Center. More detailed information can be found here.
Tableau Cloud Release Preview
Under GxP guidance, SaaS providers are expected to provide the SaaS application users access to an environment where users can assess the impact of upcoming major functional changes, perform any necessary regression testing, and train users before such changes are applied to regulated environments.
Tableau Cloud now provides Tableau+ Edition customers the unique ability to create and maintain Release Preview sites that receive major functional changes a minimum of 2 weeks earlier than other Tableau Cloud sites. Companies with GxP requirements can leverage Release Preview sites to qualify and validate ahead of regular updates. As of April 2025, Tableau Cloud administrators with Tableau+ Edition can create and manage Release Preview sites using Tableau Cloud Manager.
Access to Release Preview provides regulated companies with environments to conduct the impact analysis, regression testing, and essential training that is necessary for maintaining their GxP compliance.
The Tableau Release Navigator dashboard captures the functional changes between the current Tableau Cloud release and that on the Release Preview Sites. This provides customers with a quick reference for understanding functional changes.
Roles and Responsibilities
It’s important to have clearly defined roles in the Tableau Cloud migration process:
Infrastructure team
This team is responsible for qualifying Tableau Cloud. A business or system owner for Tableau may not be well-versed in clinical development or regulatory affairs, but they are responsible for the Tableau Cloud platform and will ensure its qualification. This team will interact with the compliance team around qualification and its documentation.
Compliance team
The compliance team is responsible for overseeing the entire compliance program. The team develops and implements compliance policies and procedures, ensures all activities comply with GxP regulations, conducts regular audits and risk assessments, and provides training and support to staff on compliance matters.
Organizational Change Management team
Organizational Change Management (OCM) team has several key duties to ensure smooth transitions and compliance with regulatory standards, one of them being to provide training and support to employees to help them transition smoothly through the change process.
Computer System Validation team
The Computer System Validation3 (CSV) team has several critical duties to ensure that computerized systems comply with regulatory standards and function as intended including but not limited to Requirement specifications, Change Management, and Continuous Improvement.
Quality Assurance team
The Quality Assurance (QA) team oversees overall compliance and quality across all processes. The QA team is responsible for maintaining comprehensive documentation, including Standard Operating Procedures (SOPs), training records, and audit reports. Other activities include audits, training, and incident management.
BI & Analytics team
The BI & Analytics team is responsible for platform enablement of all users and any communication related to the migration project to ensure a smooth transition and high adoption.
GxP Validator
This role doesn’t have to be in the IT team or the business using Tableau, but someone responsible for ensuring GxP compliance is being met.
Getting GxP-Ready on Tableau Cloud
Achieving Tableau Cloud qualification is a crucial first step, as it involves ensuring the platform’s functional use is thoroughly tested. The initial maturity level focuses on reaching the qualification layer, which builds collective confidence in the platform’s performance from a SaaS perspective, as well as in terms of privacy and GCPs4. This solid foundation is essential for developing specific validation applications tailored to particular business functions, ensuring compliance and reliability.
Tableau Cloud sites are containers that Tableau administrators can use to manage workbooks, data sources, flows, projects, views, metrics, and other assets, along with permissions (Users and Groups) to allow the HLS organization members to use the resources.
A common practice is to isolate GxP environments. To simplify solution management, the best practice is to have team-specific development environments. Test and Production environments can be shared by many teams. To ensure data privacy you may also decide to leverage multiple pods regions (EMEA, USA, Asia, etc).
Out of the 50 Tableau Cloud sites available (part of the Tableau+ license), the minimum should be:
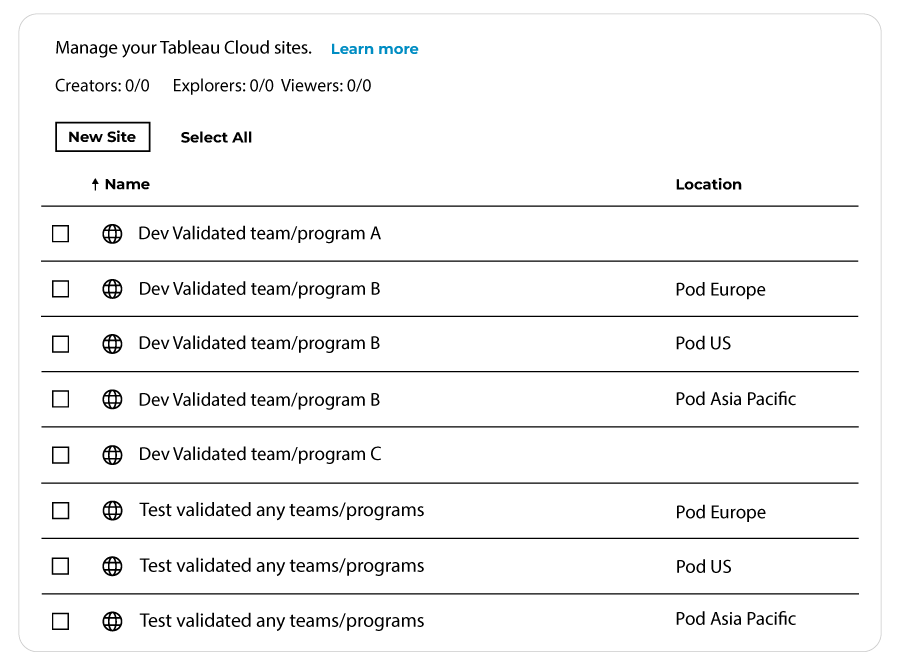
An alternative option is to provide the business teams with one site and manage environments (dev, test, and prod) creating relevant Tableau projects (a kind of folder structure) within that said site:
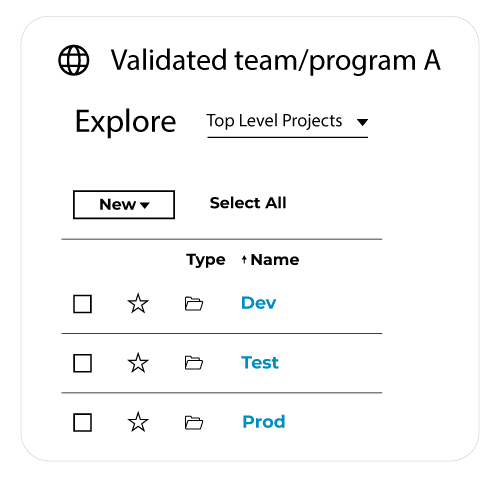
Another important activity to implement is the synchronization between your production sites and release preview sites. This includes synchronizing content (such as dashboards and data sources), connectivity, users, and related security.
Setting up Tableau Cloud sites is an essential step before migrating and then creating validated applications. This process lays the groundwork, with the Tableau Cloud setup serving as the initial building block. Once these elements are in place, teams can migrate and then construct the rest of their non-validated and validated applications within the environment.
To monitor your Tableau Cloud sites, you have the following resources and tools available:
- Administrative Views: These are built-in dashboards that help you understand system utilization and user interactions. They provide insights into background tasks, data source traffic, and more.
- Tableau Cloud Site Capacity: This resource helps you understand the various capacities of your Tableau Cloud sites, and how you can monitor your usage.
Tableau Cloud Migration Waves – GxP Qualification and Validation Frameworks
There are plenty of online resources on how to successfully migrate from Tableau Server to Tableau Cloud, but in this context, you need to define a clear Tableau Cloud migration plan that considers the GxP constraints. What is unique to GxP-related Tableau Cloud migrations is the need to add to standard Tableau Cloud migration projects some qualification and validation frameworks.
Due to GxP constraints, you will need to ensure compliance not only during the migration and at the migration signoff but also on an ongoing basis to keep the platform compliant. This is not a one-time project; it’s an ongoing effort. One of the best practices is to patch Tableau Server to the closest Tableau Cloud release before starting with the migration to minimize potential issues.
While starting to qualify Tableau Cloud, you need to start migrating non-validated projects (such as commercial, marketing, etc) while implementing the foundation to ease the migration of the validated projects. You can follow all the best practices and methodologies you need to start migrating in the first migration wave with a first project/team eager to embrace Tableau Cloud innovations and associated changes.
Depending on the size of your Tableau Server deployment you may need to run multiple non-validated migration waves before migrating the validated ones. It will give you plenty of time to focus in parallel on implementing the necessary GxP frameworks.
The Tableau Cloud migration project plan should look like this:
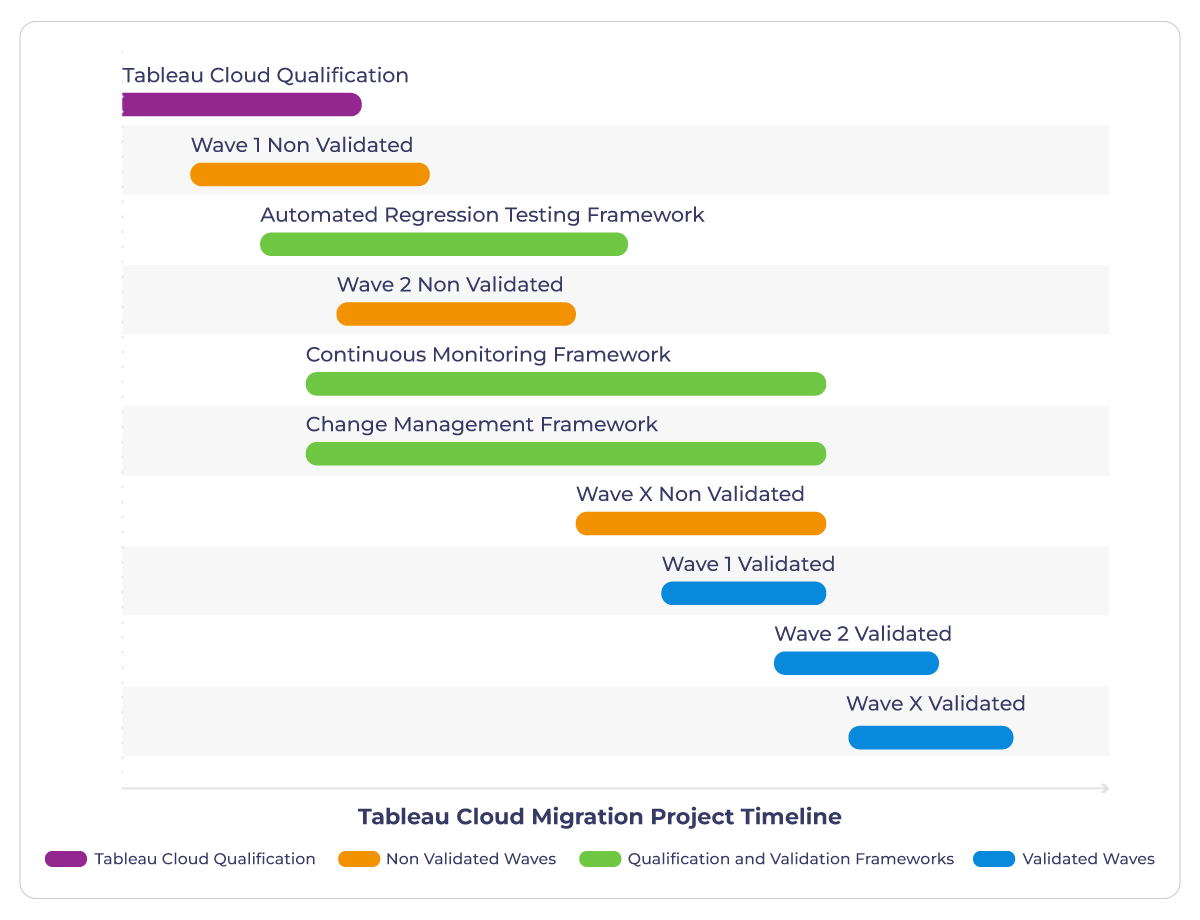
In the highly regulated and scrutinized world of HLS, companies must be prepared for inspections by health authorities and regulatory bodies thus they should focus on 3 qualification and validation frameworks to implement:

Framework 1
Automated Testing and Related Audit Trails

Framework 2
Continuous Monitoring and Related Audit Trails

Framework 3
Change Management and Related Audit Trails
You need to design (or adapt existing ones for Tableau Server) these frameworks right after initiating the first non-validated migration wave. This approach allows you to benefit from them immediately, even for non-validated content. More importantly, it enables you to perform a dry run with a non-validated wave, ensuring readiness for the validated waves. The goal is to establish the Analytics Governance policies you will implement.
What is common among these three frameworks is their ability to generate and store associated audit trails, following best practices such as maintaining audit trails for the lifetime of a patient and ensuring they are time-stamped. More information on the key characteristics of an audit trail can be found here. Those audit trails, along with all versions of your test plans, test runs/results, defects/deviations, requirements, and sign-offs, should be stored within your quality/qualification solution, such as Veeva, ALM, or any other solution you are using.
Framework 1: Automated Testing and Related Audit Trails for Qualification and Validation
There are different types of tests that need to be performed during the Tableau Cloud migration project and afterward to maintain compliance on the Tableau Cloud Platform (run mode). Those tests will be used for the qualification and validation of the Tableau Cloud Platform.
1. Qualification
The most important type of test here is side-by-side regression testing; this validates that the same content has no change between Tableau versions. This includes visual, worksheet data and metadata differences, and functional aspects such as performance. The goal is to make sure there are no defects and that there are no performance gaps.
This is probably the most critical policy to enforce. It will be useful for:
- Migration from Tableau Server to Tableau Cloud: You need to perform side-by-side regression testing to validate that the same content that existed in Tableau Server now exists in Tableau Cloud and that there is no change. This must be performed throughout the Tableau Cloud migration waves and will prove the success of your migration.
- Testing Tableau Cloud Release Previews: Tableau will provide release previews for qualification and validation purposes . You will need to perform regression tests comparing your content on both your Tableau Cloud production sites and release preview sites. For HLS companies, timing and speed are critical. The faster you complete qualifications, the more time you have to address potential defects (bugs, regressions, side effects). Thorough documentation is essential. If a defect is identified, it must be addressed, and you may need to requalify the impacted dashboards or the release preview, ensuring continuous compliance.
- Testing Tableau Cloud Upgrades: Despite release preview testing efforts or if you are not licensed for it (Tableau +), you still need to perform regression tests between the Tableau Cloud production sites hosting your validated content before and after the upgrades happen. You can also expand this concept to test two release preview versions.
The testing requirement documents you need to write for these tests (regression testing) are very basic and similar between those 3 scenarios. You may need to perform regression testing in bulk. You may currently have a strategy on Tableau Server to identify defects in a small set of dashboards and then perform impact analysis to determine which dashboards are affected by the same defects. However, running regression tests on all your dashboards allows you to adopt a more comprehensive approach. Essentially, this means testing all your dashboards to see what works.
2. Validation
It’s important to design comprehensive testing packages to ensure business applications function correctly and dashboard outputs are accurate. A critical test is verifying completeness and accuracy by comparing trusted data from a validated source with the dashboard data, effectively validating the last mile of the data journey. Ensure dashboards reflect underlying data accurately to meet user requirements. Testing and verification should align with the risk associated with GxP data, patients, and processes, providing evidence of data integrity, security, and confidentiality.
The FDA calls this System Dependability: “The sponsor should ensure and document that computerized systems conform to the sponsor’s established requirements for completeness, accuracy, reliability, and consistent intended performance.”
Focus on the quality of dashboards and underlying Tableau data sources, ensuring completeness, accuracy, and reliability. This is crucial for self-service analytics and Tableau AI capabilities, which depend on robust data sources. The testing requirement documents for these validation tests are more complex than those for regression testing.
The primary goal is to achieve qualification and validation as quickly as possible. You need to leverage automation allowing you to create reusable components, enabling businesses to derive value swiftly and efficiently. Automation will allow you to:
- Expedite testing to allocate more time for resolving potential defects.
- Perform automated and bulk testing to eliminate the need to ask Business Units to form testing or validation teams.
You can decide whether you want to apply those testing automation packages to non-validated content. At a minimum, you need to dry run them on non-validated content as well as use them for mission-critical dashboards.
Framework 2: Continuous Monitoring and Related Audit Trails for Qualification and Validation
You need to conduct periodic reviews of Tableau Cloud artifacts to ensure they remain in a validated state, which involves constant validation or revalidation of the systems. Automated testing during migrations, upgrades, or to validate Release Previews is not sufficient. In a Cloud environment, continuous monitoring and testing are essential. Tableau Cloud versions can be updated for security reasons (e.g., critical CVEs) without warning, and underlying components such as your Cloud database (e.g., Snowflake) or ETL/ELT tools may also be updated.
You need to automate this monitoring. The iterative approach is crucial—continuously thinking about how each business function can benefit from this technology as soon as possible is always the key.
You need to constantly monitor performance as it is part of a GxP requirement. It is a paradigm shift between Tableau Server and Tableau Cloud. While running Tableau Server on-premise, you have a team tracking server performance, making it easy to adapt your infrastructure as you scale. However, running Tableau Cloud requires a different approach. You need to monitor performance evolution and trends continuously.
Consider the example of Dashboard A. When migrating Dashboard A from Tableau Server to Tableau Cloud, you need to ensure, through regression testing, that its performance is equal to or better than before. This applies to comparisons between a Tableau Cloud version and a Tableau Cloud Release Preview, as well as between the current Tableau Cloud version and future releases (including upgrades, even if tested during the Release Preview period). Additionally, you must monitor the performance of Dashboard A over time to track its evolution. Similarly, for validation test packages, you need to run them continuously to proactively detect any defects.
Framework 3: Change Management and Related Audit Trails for Qualification and Validation
This is the final workflow to implement and should be implemented by the infrastructure team, and used by the business teams for validated sites or projects (as well as mission-critical content).
Putting in place the two previous workflows allows you to keep control over your Tableau Cloud environment, but you also need to set up a solid Tableau content lifecycle management. You may already have a similar workflow in place on Tableau Server that you need to adapt as the Tableau Cloud production sites will be flooded with new content or new versions of existing content.
Imagine a pool with crystal-clear water that you maintain through rigorous testing and continuous monitoring (the two previous frameworks). If you have a hose filling this pool, it’s crucial to ensure the hose also meets the same quality standards. Otherwise, despite all your efforts, the situation will quickly become unmanageable.
The pool represents your qualified and validated Tableau Cloud artifacts, while the hose water symbolizes your new dashboards (or data sources) or new versions of existing dashboards (or data sources). This content needs to undergo a series of tests, especially performance tests, both in the QA environment and, more importantly, in the Production environment. As with previous workflows, tracking performance is paramount when running a cloud platform.
A version should be maintained for each new dashboard or new version of an existing dashboard, along with an audit trail of the dashboard’s lifecycle: when it was created, who created it, who put it into production, and who approved it. For any new version, document when it was modified, who modified it, what the modification was, and who approved it. You cannot rely solely on Tableau’s built-in revision history capabilities, as they are limited to 10 versions in Tableau Cloud and allow for the deletion of revisions.
Implementing robust change management practices, including version control, is essential to track modifications and maintain the integrity of systems.
With these three Analytics Governance frameworks in place, you will be able to continuously qualify your Tableau Cloud platform and validate the business applications built on it. This will pave the way for successfully embracing Tableau AI capabilities while reinforcing your GxP compliance. These frameworks should be reviewed and documented by the QA team and the validation team.
The Benefits of GxP Compliance
Seeing Beyond the Technical GxP Qualification and Validation: Having a Business Impact
In the biotech and pharma industries, it’s crucial for IT teams to focus on business outcomes as part of their role. Clear communication about the decisions you aim to expedite, whether in clinical or safety areas, is essential. Streamlining processes can lead to significant cost reductions, particularly in clinical trials where delays can cost hundreds of millions of dollars. Demonstrating the business benefits of faster transitions, such as reducing the time to move from phase one to phase two by six months, can be transformative. Building relationships with passionate stakeholders and providing proof of concept can highlight value and drive momentum. Ultimately, securing endorsement from both IT and business leaders, including clinicians, is key to achieving success. Infrastructure teams must not only ensure technical excellence but also demonstrate the tangible business value of their efforts.
Read 👉 The Critical Role of Data Analytics Quality for Clinical Research
Seeing Beyond GxP Compliance
Once you have mastered GxP compliance, you can leverage the best practices and methodologies described in this white paper for other regulations, such as SOX reporting. In the pharmaceutical industry, compliance extends beyond GxP to include privacy, healthcare compliance, and SOX, to name a few. The largest companies in these highly regulated sectors must ensure comprehensive compliance across all these areas.
Other industries, such as financial institutions (including Financial Services, Banking, and Insurance), can apply the best practices, methodologies, and workflows outlined in this white paper for risk reporting, financial reporting, and other regulatory requirements, including BCBS 239 and SOX compliance.
Ultimately, the principles and practices can cascade to other sectors facing regulatory challenges, demonstrating the versatility and broad applicability of these compliance strategies. They can also benefit any company looking to enhance their Tableau Cloud or Tableau Server Enterprise Readiness capabilities.
Why Partner with Wiiisdom
By adopting innovative Wiiisdom solutions from the outset, HLS companies can achieve quick, impactful wins throughout the entire data journey, from analytics consumption (data sources and dashboards) to Tableau AI insights, thereby reinforcing a culture of quality and informed decision-making. This enables the delivery of analytics and insights in a validated state much more effectively.
Leveraging Wiiisdom solutions, HLS companies can expedite qualification and validation processes efficiently. They can utilize Wiiisdom’s unique expertise to build reusable components that deliver value swiftly. Wiiisdom’s iterative approach ensures that each business function can reap the benefits of the technology as quickly as possible. This is key to maximizing efficiency and achieving rapid results.
Wiiisdom is partnering with the largest pharmaceutical companies in the world to address GxP while running Tableau Cloud (as well as Tableau Server), serving tens of thousands of Tableau users. Wiiisdom is also assisting major regulators, including the FDA. Wiiisdom helps these organizations to achieve regulatory compliance with comprehensive traceability and detailed records.
As a trusted Tableau technology partner, Wiiisdom collaborates with Tableau to help HLS clients achieve GxP compliance on Tableau Cloud, with its announcement of the release of new Tableau version management capabilities.
Wiiisdom’s Analytics Governance solutions provide documentation, validation details, predictive monitoring capabilities, and change management to ensure complete control over Tableau Cloud. Wiiisdom is named for the second year as a Sample Vendor in the Gartner® Hype Cycle™ for Data and Analytics Governance 2024 and 2025.
Partnering with Wiiisdom offers significant benefits to the HLS industry, which is eager for innovation and faster progress. Unlike other industries where an 80/20 approach might suffice, HLS companies need to ensure absolute accuracy and reliability of their data, especially in the last mile within the analytics layer. Wiiisdom’s expertise and solutions can help meet these high standards, enabling HLS companies to achieve the efficiency they desire while maintaining rigorous data validation and compliance. Ultimately, Wiiisdom is helping HLS companies to embrace corporate strategies around embracing GenAI analytics.
When Innovation Meets Innovation
Just as leading pharmaceutical companies are at the forefront of innovation, we at Wiiisdom take pride in our pioneering patent-pending technologies. Our mission is to enhance the data consumption experience for organizations. In the same way that we rely on reviews, trust marks, and labels in our daily lives to make informed decisions, data consumers should have similar assurances regarding their Business Intelligence (BI) content. A visual label or stamp of quality reassures users that the data that supports the decision-making process is reliable and trustworthy.
When data consumers open their dashboards, they will be able to trust the data presented, thanks to our automated and dynamic certification capabilities. This certification confirms that the data is suitable for decision-making. Our technology transforms organizations from a reactive approach to a proactive one as it allows them to quickly identify defects and inform the appropriate teams for resolution showcasing our game-changing and patent-pending technology.
Given that pharmaceutical companies consistently strive for the highest standards in governance and quality, they should also embrace dynamic and proactive certification processes. This approach serves their business by providing a trust mark on validated dashboards and ensures that they remain at the cutting edge of industry standards. Continuously improving validation processes to enhance efficiency and compliance is part of the CSV team’s responsibilities and aligns with Wiiisdom’s innovative approach.
Seeing Beyond the Tableau Cloud Migration: Embrace GenAI Analytics
The loop is completed when you fully embrace Tableau’s Cloud and AI innovations and effectively align them with your corporate strategy. Once you have moved to Tableau Cloud, it’s time to leverage technologies like Tableau Pulse and Tableau Agent. However, the need for Analytics Governance becomes even more critical with these technologies. Remember that, like any AI engine, Tableau AI is only as good as the data fueling it. Success lies in ensuring the reliability of the insights, as they often inform critical business decisions and strategies.
Gartner states, “With growing regulation tied to AI, analytics governance is now required, not a nice-to-have.” 1
1.Gartner, Hype Cycle for Data and Analytics Governance, 2024, By Guido De Simoni, Andrew White, Saul Judah, 18 June 2024
GARTNER is a registered trademark and service mark of Gartner, Inc. and/or its affiliates in the U.S. and internationally and is used herein with permission. All rights reserved.
Gartner does not endorse any vendor, product or service depicted in its research publications, and does not advise technology users to select only those vendors with the highest ratings or other designation. Gartner research publications consist of the opinions of Gartner’s research organization and should not be construed as statements of fact. Gartner disclaims all warranties, expressed or implied, with respect to this research, including any warranties of merchantability or fitness for a particular purpose.
With GenAI analytics, turning data into insights can be achieved at the speed of light, making manual certification and manual validation obsolete.
Wiiisdom provides governance capabilities to ensure trusted GenAI insights around the clock. These automated and dynamic capabilities are essential for organizations looking to fully embrace AI innovations while aligning with their corporate strategy and maintaining high data integrity and governance standards.
HLS companies are well-positioned to successfully adopt Tableau AI due to their unwavering commitment to quality, fostered by GxP guidelines. While some may view GxP as a constraint, for HLS companies, it is an invaluable asset that ensures high-quality analytics assets, thereby minimizing AI hallucinations, particularly in Tableau data sources, which are central to many of Tableau AI’s innovations.
Conclusion
AI capabilities are set to become essential for all pharmaceutical organizations due to their transformative potential. By leveraging AI and the Cloud, HLS companies can harness advanced analytics to architect, enrich, and transport innovative ideas, creating significant value. This technology enables continuous learning and rapid adaptation. The shift towards AI in the Cloud ensures that pharmaceutical organizations remain competitive and at the forefront of medical advancements. Embracing Tableau Cloud AI innovations alongside Wiiisdom’s innovative solutions to ensure GxP compliance is the way forward for HLS companies.
Terms and Definitions
1 System Qualification: System qualification is the process of demonstrating that a system is properly installed, operates correctly, and produces the expected results. This typically involves Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) to ensure that the system meets all regulatory and operational requirements.
2 Change Management: Change management in the context of GxP compliance refers to the systematic approach to managing changes to a system or process. This includes evaluating the impact of changes, obtaining necessary approvals, implementing the changes in a controlled manner, and documenting the entire process to ensure compliance with regulatory requirements.
3 System Validation: System validation is the documented process of ensuring that a computerized system performs as intended in a consistent and reproducible manner. This involves a series of activities, including planning, testing, and documentation, to confirm that the system meets all specified requirements and regulatory standards.
4 GCP: Good Clinical Practice (GCP) is an international quality standard and covers the way a clinical trial is designed, conducted, performed, monitored, audited, recorded, analyzed, and documented.

